Abstract
Background:
Measurable residual disease (MRD) status following complete remission (CR) is correlated with the survival of patients (pts) with B-cell acute lymphoblastic leukemia (ALL). Among pts achieving CR with standard therapy, the persistence or recurrence of MRD is associated with a high relapse rate and poor outcomes. MRD-directed therapy with the bispecific T-cell engager blinatumomab, has been investigated, although confirmatory studies are needed.
Methods:
This is a phase II, single-arm trial of pts with B-cell ALL in CR who did not achieve MRD negativity or who relapsed with positive MRD after ≥3 months from the start of frontline therapy (CR1) or 1 month from the start of any salvage therapy (CR2 and beyond). MRD positivity was defined as ≥0.01% by multiparameter flow cytometry for pts with Philadelphia chromosome (Ph)-negative ALL, and BCR-ABL/ABL1 transcript ratio of ≥0.01% by PCR in pts with Ph-positive ALL. Negative MRD was defined as undetectable MRD by flow and PCR at a sensitivity of at least 10 -4. Blinatumomab was given at standard doses (up to 28 µg/day) for 4 weeks on and 2 weeks off (42-day cycles) for up to 5 total consolidation cycles. The decision to proceed with allogeneic stem cell transplantation (ASCT) depended on donor availability and pt fitness. Pts who did not undergo ASCT were offered maintenance therapy with blinatumomab one cycle every 3 months for up to 4 maintenance cycles (9 cycles total). All pts with Ph-positive ALL had a TKI added to their regimen.
Results:
Thirty-nine pts were treated with blinatumomab for MRD-positive B-cell ALL between December 2015 and July 2021; 25 pts (59%) for persistent MRD and 14 pts (41%) for MRD relapse. Thirty-four pts were evaluable for response. Median age was 43 years (range, 22-84). Twenty-six pts (76%) were treated in CR1 and 8 pts in CR2 and beyond (24%). Fifteen pts (44%) had Ph-positive ALL and received TKIs in addition to blinatumomab: 12 pts had ponatinib, 2 had dasatinib and 1 had imatinib.
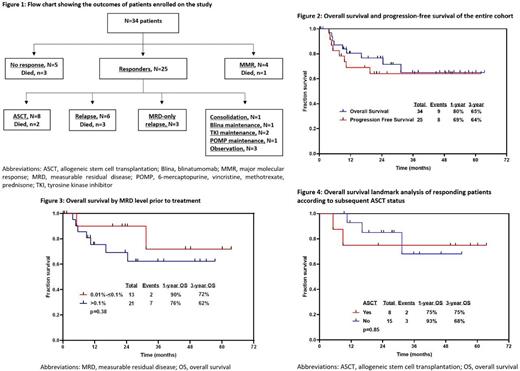
Twenty-five patients (74%) achieved MRD negativity following blinatumomab consolidation, which was given for a median of 3 cycles (range, 1-5). Twenty-three of 25 responders (92%) achieved MRD negativity after 1 cycle, 1 after 2 cycles and 1 after 4 cycles. MRD negativity by flow cytometry was achieved among 16 of 19 pts (84%) with Ph-negative ALL, and by PCR among 9 of 15 pts (60%) with Ph-positive ALL. Four additional pts with Ph-positive ALL achieved major molecular response as best response. Eighteen of 26 pts in CR1 achieved MRD negativity (69%) compared to 7 of 8 pts in CR2 and beyond (88%) (P=0.403). Three pts received blinatumomab maintenance, all in CR1. At a median follow-up of 24 months (range, 19-28), 2 of those 3 pts had MRD-only relapse. Eight pts (32% of MRD responders) underwent ASCT (5 in CR1, 2 in CR2 and 1 in CR3) with median time to ASCT of 3 months (range, 2-6).
At a median follow-up of 28 months (range, 1-63), 6 pts have relapsed (24% of MRD responders) (Figure 1). Four pts had hematologic relapse, 1 pt had hematologic plus CNS relapse and 1 pt had extramedullary relapse. All 6 relapses occurred in patients who did not undergo ASCT (2 in CR1 and 4 in CR2 and beyond). The 3-year overall survival (OS) and progression-free survival (PFS) rates for the entire cohort were 65% and 64%, respectively (Figure 2). The 3-year OS rate for pts with an MRD level at enrollment of 0.01%-0.1% (n=13) and >0.1% (n=21) were 72% and 62%, respectively (P=0.38) (Figure 3). In a landmark analysis among responding patients, 3-year OS rates were comparable between patients who underwent subsequent ASCT and those who did not (75% and 68%, respectively, P=0.85) (Figure 4).
Blinatumomab was associated with related adverse events in 16 pts (47%). These consisted of cytokine release syndrome in 4 pts (grade 3, n=1; grade 2, n=2; grade 1, n=1), confusion in 3 pts (grade 2, n=2; grade 3, n=1), headache in 3 pts (grade 2, n=2; grade 1, n=1), fever in 2 pts (grade 1), altered mental status in 2 pts (grade 3), and encephalopathy (grade 3) and psychosis (grade 3) in 1 pt each. Treatment discontinuation due to side effects occurred in 2 pts (grade 3 neurotoxicity).
Conclusion:
Blinatumomab consolidation is highly effective in pts with B-cell ALL having persistent MRD or experiencing MRD relapse after intensive chemotherapy, which may be used as definitive treatment or as a bridge to transplant. Future studies are warranted to better identify the subset of patients benefiting most of ASCT following blinatumomab therapy.
Kantarjian: Pfizer: Honoraria, Research Funding; Ipsen Pharmaceuticals: Honoraria; KAHR Medical Ltd: Honoraria; Ascentage: Research Funding; Jazz: Research Funding; Novartis: Honoraria, Research Funding; Astellas Health: Honoraria; AbbVie: Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Astra Zeneca: Honoraria; Immunogen: Research Funding; Daiichi-Sankyo: Research Funding; BMS: Research Funding; Aptitude Health: Honoraria; NOVA Research: Honoraria; Precision Biosciences: Honoraria; Taiho Pharmaceutical Canada: Honoraria. Short: Astellas: Research Funding; AstraZeneca: Consultancy; Jazz Pharmaceuticals: Consultancy; NGMBio: Consultancy; Takeda Oncology: Consultancy, Research Funding; Novartis: Honoraria; Amgen: Consultancy, Honoraria. Konopleva: F. Hoffmann-La Roche: Consultancy, Honoraria, Other: grant support; AbbVie: Consultancy, Honoraria, Other: Grant Support, Research Funding; AstraZeneca: Other: grant support, Research Funding; Rafael Pharmaceuticals: Other: grant support, Research Funding; Sanofi: Other: grant support, Research Funding; KisoJi: Research Funding; Cellectis: Other: grant support; Novartis: Other: research funding pending, Patents & Royalties: intellectual property rights; Ascentage: Other: grant support, Research Funding; Forty Seven: Other: grant support, Research Funding; Agios: Other: grant support, Research Funding; Reata Pharmaceuticals: Current holder of stock options in a privately-held company, Patents & Royalties: intellectual property rights; Stemline Therapeutics: Research Funding; Calithera: Other: grant support, Research Funding; Genentech: Consultancy, Honoraria, Other: grant support, Research Funding; Ablynx: Other: grant support, Research Funding; Eli Lilly: Patents & Royalties: intellectual property rights, Research Funding. Jain: Incyte: Research Funding; Aprea Therapeutics: Research Funding; Fate Therapeutics: Research Funding; Janssen: Honoraria; Servier: Honoraria, Research Funding; Pfizer: Research Funding; Cellectis: Honoraria, Research Funding; Bristol Myers Squibb: Honoraria, Research Funding; ADC Therapeutics: Honoraria, Research Funding; Adaptive Biotechnologies: Honoraria, Research Funding; Precision Biosciences: Honoraria, Research Funding; Pharmacyclics: Research Funding; TG Therapeutics: Honoraria; Beigene: Honoraria; Genentech: Honoraria, Research Funding; AbbVie: Honoraria, Research Funding; AstraZeneca: Honoraria, Research Funding. Ravandi: Agios: Honoraria, Research Funding; Prelude: Research Funding; Jazz: Honoraria, Research Funding; Novartis: Honoraria; Xencor: Honoraria, Research Funding; AbbVie: Honoraria, Research Funding; Taiho: Honoraria, Research Funding; Astex: Honoraria, Research Funding; AstraZeneca: Honoraria; Amgen: Honoraria, Research Funding; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Syros Pharmaceuticals: Consultancy, Honoraria, Research Funding. Daver: Amgen: Consultancy, Research Funding; Daiichi Sankyo: Consultancy, Research Funding; Bristol Myers Squibb: Consultancy, Research Funding; Trillium: Consultancy, Research Funding; Novimmune: Research Funding; Glycomimetics: Research Funding; ImmunoGen: Consultancy, Research Funding; FATE Therapeutics: Research Funding; Pfizer: Consultancy, Research Funding; Gilead Sciences, Inc.: Consultancy, Research Funding; Hanmi: Research Funding; Trovagene: Consultancy, Research Funding; Sevier: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; Astellas: Consultancy, Research Funding; Abbvie: Consultancy, Research Funding; Jazz Pharmaceuticals: Consultancy, Other: Data Monitoring Committee member; Novartis: Consultancy; Dava Oncology (Arog): Consultancy; Celgene: Consultancy; Syndax: Consultancy; Shattuck Labs: Consultancy; Agios: Consultancy; Kite Pharmaceuticals: Consultancy; SOBI: Consultancy; STAR Therapeutics: Consultancy; Karyopharm: Research Funding; Newave: Research Funding. Alvarado: Jazz Pharmaceuticals: Research Funding; Daiichi-Sankyo: Research Funding; CytomX Therapeutics: Consultancy; BerGenBio: Research Funding; FibroGen: Research Funding; MEI Pharma: Research Funding; Astex Pharmaceuticals: Research Funding; Sun Pharma: Consultancy, Research Funding. Takahashi: GSK: Consultancy; Symbio Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene/BMS: Consultancy; Novartis: Consultancy. Jabbour: Amgen, AbbVie, Spectrum, BMS, Takeda, Pfizer, Adaptive, Genentech: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal